Answer:
Step-by-step explanation:
The chemical formula of Benadryl is
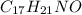
1 mol of Benadryl has 17 mol of hydrogen
2.5 mol of Benadryl has 17*2.5 = 42.5 moles of hydrogen.
Mols are peculiar chemical definitions. You have to be careful what you are talking about. You are given mols of Benadryl, which is the whole molecule. Whole molecule means the Carbon Hydrogen Nitrogen and Oxygen
Each mol of Benadryl contains 17 mols of carbon, 21 moles of hydrogen, 1 mol of nitrogen and 1 mol of oxygen