Answer:
The molarity of the diluted solution will be 0.208 M.
Step-by-step explanation:
Dilution consists of a procedure to prepare a less concentrated solution from a more concentrated one. It is achieved by adding more solvent to the same amount of solute.
The quantity or mass of the solute is not changed but only that of the solvent. As only solvent is being added, the consequence is that by not increasing the amount of solute but if the amount of solvent, the concentration of the solute decreases.
The expression for a dilution is:
Ci * Vi = Cf * Vf
where
- Ci: initial concentration
- Vi: initial volume
- Cf: final concentration
- Vf: final volume
In this case:
- Ci= 0.25 M
- Vi= 125 mL
- Cf= ?
- Vf= Vi + 25 mL= 125 mL + 25 mL= 150 mL
Replacing:
0.25 M* 125 mL= Cf* 150 mL
Solving:
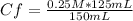
Cf= 0.208 M
The molarity of the diluted solution will be 0.208 M.