Answer:
Sample equal to the atomic mass of an element in grams contains 6.02214179 × 10²³ atoms. This amount of matter is called a mole
Step-by-step explanation:
Experimentally, it has been determined that 1 mole of any substance contains 6.02214179 × 10²³ elementary entities. The number of elementary entities in the mole is known as Avogadro's number,
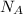 , or the Avogadro's constant and the unit of the constant is per mole
, or the Avogadro's constant and the unit of the constant is per mole
The molar mass of a compound or an element is the mass of 1 mole of the compound or the element given in grams
Similarly, the gram atomic mass is the mass in grams of 1 mole of the atoms of the element that contains one Avogadro's number of atoms
Therefore, we have;
The number of atoms in a sample equal to the atomic mass of an element in grams = 6.02214179 × 10²³ atoms
The name given to the amount of matter containing equivalent to 6.02214179 × 10²³ elementary particles = A mole.