Answer:
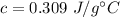
Step-by-step explanation:
The increase in heat, Q = 58.1 J
Mass, m = 89.4 g
The temperature increases from 23.4°C to 25.5°C. We need to find the specific heat of an unknown metal.
We know that the increase in heat is given by :
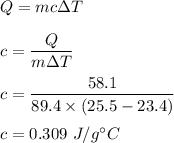
So, the specific heat of an unknown metal is equal to
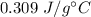 .
.