Answer:
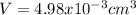
Step-by-step explanation:
Hello there!
In this case, according to the given atoms of aluminum, it is possible for us to calculate the mass via the Avogadro's number and its atomic mass of 27.0 g/mol:
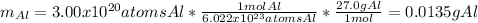
Noe, given the density of the Al, we can calculate the volume as shown below:
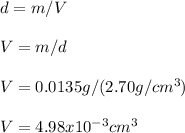
Best regards!