Answer:
Following are the responses to the given choices.
Step-by-step explanation:
For point a:
Its idea of the test is to calculate water through unknown hydrate that has been evaporated on the burn.
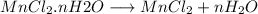
This mole ratio is abnormal to the sample amount of water vaporized. Each student is thus able to examine only 1 g of sample. The amount of water is then linked to a standard table that she has already planned.
Its empirical definition of
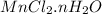 to n variation of 1 to 5 was distinct and the water percentage was determined by mass. If higher the amount of hydrate, the higher the amount of water in salt.
to n variation of 1 to 5 was distinct and the water percentage was determined by mass. If higher the amount of hydrate, the higher the amount of water in salt.
Its results vary from
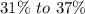 of water content. Its water slides for three moisturize molecules are
of water content. Its water slides for three moisturize molecules are
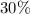 and the water slides for four hydrate molecules are
and the water slides for four hydrate molecules are
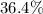 . Her argument which four hydrate molecules could therefore be eliminated could be inaccurate.
. Her argument which four hydrate molecules could therefore be eliminated could be inaccurate.
For point b:
The error could be incomplete fire, which could not have vaporized all hydrate compounds.Its student may not have evaporated all swimming pools in the salt, because the estimate of their moisture content could be on the bottom.
Its average moisture content is roughly
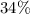 . Consequently, it should logically search for water content close to over
. Consequently, it should logically search for water content close to over
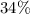 . In addition to the average value, thus, the salt is composed of 4 moisturize molecules of approximately
. In addition to the average value, thus, the salt is composed of 4 moisturize molecules of approximately
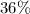 .
.