Answer:
0.033 moles of KCl are formed.
Step-by-step explanation:
The reaction that takes place is:
To solve this problem we need to convert 0.05 moles of O₂ into moles of KCl, to do so we use the stoichiometric coefficients of the balanced reaction:
- 0.05 mol O₂ *
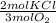 = 0.033 mol KCl
= 0.033 mol KCl
If 0.05 moles of O₂ are produced, then 0.033 moles of KCl are formed as well.