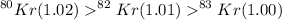Solution :
According to the Graham's law of diffusion, we know that, the rate of the diffusion varies inversely to the molar mass of the gas, i.e.
Rate of diffusion,
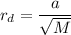
where, the 'M' is the molar mass of the gas.
Now in the case of the isotopes of the Krypton,
Atomic mass of
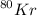 = 80 AMU
= 80 AMU
Atomic mass of
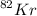 = 82 AMU
= 82 AMU
Atomic mass of
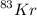 = 83 AMU
= 83 AMU
So the ratio of the rate of diffusion of the three isotopes are :
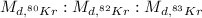
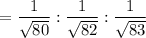
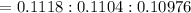
Dividing the above three with the smallest number among the three i.e. 0.10976, we get the relative rates of diffusion.
∴
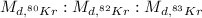
= 1.02 : 1.01 : 1
Hence the relative rate of diffusion are :