Answer:

Step-by-step explanation:
To covert from grams to moles, the molar mass is used. This is the mass of 1 mole of a substance. These are located on the Periodic Table. They are the equivalent to the atomic masses, but have different units: grams per mole.
We are given the compound: NaNO₃
Look up the values for each individual element (sodium, nitrogen, and oxygen)
- Na: 22.989769 g/mol
- N: 14.007 g/mol
- O: 15.999 g/mol
Now find the molar mass of the entire compound. Notice that oxygen has a subscript of 3 in the formula, so there are 3 atoms of oxygen per molecule. We must multiply its molar mass by 3 before adding the others.
- O₃: 15.999*3=47.997 g/mol
- NaNO₃: 22.989769+14.007+47.997=84.993769 g/mol
Use this value as a ratio.
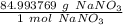
Multiply by the given number of grams: 150
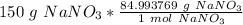
Flip the ratio so the grams of sodium nitrate cancel.
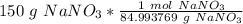
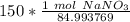
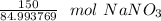
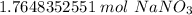
If we round to the nearest hundredth, the 4 in the thousandth place tells us to leave the 6.
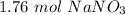
150 grams of sodium nitrate is approximately 1.76 moles of sodium nitrate. Therefore, choice A is correct.