Answer:
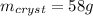
Step-by-step explanation:
Hello there!
In this case, according to the attached solubility chart, it is possible for us to realize that about 88 grams of KNO3 are soluble at 50 °C but just 30 grams are soluble at 20 °C in the same 100 g of water.
In such a way, the crystalized mass of this solute can be calculated by subtracting the mass at 50 °C and the mass at 20 °C:
Best regards!