Answer:
0.300 M
Step-by-step explanation:
- Cr₂O₇²⁻ (aq) + Sn²⁺ (aq) → Sn⁴⁺(aq) + Cr³⁺(aq)
The balanced equation is:
- Cr₂O₇²⁻ (aq) + 3Sn²⁺ + 14H⁺ (aq) → 3Sn⁴⁺(aq) + 2Cr³⁺(aq) + 7H₂O
Now we calculate how many Cr₂O₇²⁻ moles reacted, using the given volume and concentration:
- 12.50 mL * 0.0800 M = 1 mmol Cr₂O₇²⁻
Then we convert Cr₂O₇²⁻ moles into Sn²⁺ moles, using the stoichiometric coefficients of the balanced reaction:
- 1 mmol Cr₂O₇²⁻ *
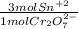 = 3 mmol Sn²⁺
= 3 mmol Sn²⁺
Finally we calculate the concentration of the Sn²⁺ species:
- 3 mmol Sn²⁺ / 10.0 mL = 0.300 M