Answer:
A) (m/Z)s / ( m/Z)u = Vu / Vs
B) 71.5
C) the ratio of accelerating voltages decrease as the value of the unknown mass increases.
Step-by-step explanation:
A) To derive the expression we have to vary one of the three variables while keeping the other two variables constant
let the variables be denoted as ; ( B , V , r )
m/z = B^2r^2e / 2v
therefore the equations can be written as
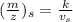 ,
,
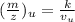
u = unknown value , s = standard value
∴ ratio of accelerating voltage to the ratio of corresponding m/Z values
= (m/Z)s / ( m/Z)u = Vu / Vs
B) find (m/Z) u
since : Vu / Vs = 0.965035
( m/Z) s = 69.00
Vu / vs = (m/Z)s / (m/Z)u -------- ( 1 )
∴ (m/Z)u = 69 / 0.965035 = 71.5
C) If the unknown mass ( m/z ) u = 143
the ratio of the voltages : Vu / vs = 69 / 143 = 0.4825
when compared to the answer gotten in b above it can be seen that the ratio of accelerating voltages decrease as the value of the unknown mass increases.