Answer:
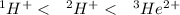
Step-by-step explanation:
Based on the given information, assuming that all three nuclear particles are isoelectronic, the greater the attraction from the nucleus, the greater the penetration. The greater the attraction to the nucleus, however, the less shielding there would be, resulting in a high effective nuclear charge.
Hence, in increasing of most penetrating to least penetrating, the arrangement is as follows.
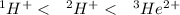
least most
penetrating penetrating