Answer:
Option D
Step-by-step explanation:
Molarity (M) of any solution is equal to the number of moles (n)of solute in it divided by total volume of solution (V)
Mathematically, it can be represented as
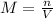
Substituting the given values, we get-
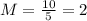
Hence, option D is correct