Answer:
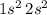 .
.
Step-by-step explanation:
Electron orbitals in an atom (e.g.,
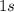 ) are denoted with:
) are denoted with:
- A number, denoting the shell (principal energy level) of this orbital, and
- A letter, denoting the shape of this orbital (
 ,
,
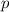 ,
,
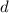 , etc.)
, etc.)
There are two aspects to consider when finding the electron configuration of an atom:
- The number of electrons that each type of orbitals could hold, and
- The order in which the orbitals are filled.
The
 orbital in each shell could hold up to
orbital in each shell could hold up to
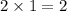 electrons (one
electrons (one
 orbital per shell, with up to two electrons.)
orbital per shell, with up to two electrons.)
The
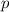 orbitals in each shell could hold up to
orbitals in each shell could hold up to
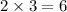 electrons (three
electrons (three
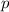 orbitals per shell, with up to two electrons in each orbital.)
orbitals per shell, with up to two electrons in each orbital.)
The
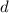 orbitals in each main shell could hold up to
orbitals in each main shell could hold up to
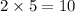 electrons (five
electrons (five
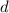 orbitals per shell, with up to two electrons in each orbital.)
orbitals per shell, with up to two electrons in each orbital.)
Refer to the order in which the orbitals are filled (Aufbau principle.)
- The first orbital to be filled would be
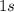 (the
(the
 orbital of the first shell,) accommodating up to
orbital of the first shell,) accommodating up to
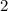 electrons.
electrons. - The second orbital to be filled would be
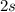 (the
(the
 orbital of the second shell,) accommodating up to
orbital of the second shell,) accommodating up to
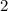 electrons.
electrons.
All four electrons of Beryllium are thus assigned to the
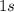 and
and
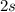 orbitals. In a ground-state Beryllium atom, orbitals
orbitals. In a ground-state Beryllium atom, orbitals
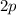 and beyond would contain no electrons.
and beyond would contain no electrons.
Notation:
- Two electrons in the
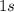 orbital:
orbital:
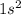 (the superscript denotes the number of electrons in this orbital (or group of orbitals).)
(the superscript denotes the number of electrons in this orbital (or group of orbitals).) - Two electrons in the
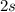 orbital:
orbital:
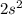 .
.
Write the non-empty orbitals in the order by which they are filled:
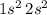 .
.