Answer:
the mass of the glycerine needed in the given solution is 23.92 g
Step-by-step explanation:
Given;
molarity of the solution (C₃H₈O₃), C = 2.60 M
Volume of the solution, V = 100 mL = 100 x 10⁻³ L = 0.1 M
The molarity of a solution is given as follows;
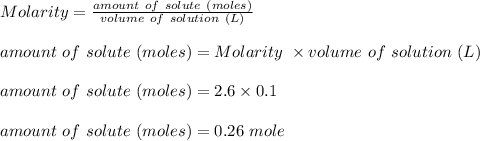
The molecular mass of the given solution;
molecular mass = (12 x 3) + (8 x 1) + (16 x 3)
molecular mass = 92 g/mol
The mass of the glycerine needed in the given solution is calculated as follows;
reacting mass = amount of solute (moles) x molecular mass (g/mol)
reacting mass = 0.26 x 92
reacting mass = 23.92 g
Therefore, the mass of the glycerine needed in the given solution is 23.92 g