Answer:
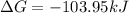
Step-by-step explanation:
Hello there!
In this case, since the thermodynamic definition of the Gibbs free energy for a change process is:
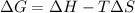
It is possible to plug in the given H, T and S with consistent units, to obtain the correct G as shown below:
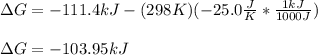
Best regards!