Answer:
The final pressure of the gas is 5 liters.
Step-by-step explanation:
Let suppose that gas experiments an isothermal process and is an ideal gas. Hence, volume is inversely proportional to pressure, that is:
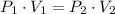 (1)
(1)
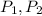 - Initial and final pressure, in atmospheres.
- Initial and final pressure, in atmospheres.
 - Initial and final volume, in liters.
- Initial and final volume, in liters.
If we know that
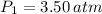 ,
,
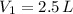 and
and
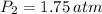 , then the final volume of the gas is:
, then the final volume of the gas is:
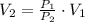

The final pressure of the gas is 5 liters.