Answer:
1540.8 Joules of energy is required to bring a 30 gram crayon from room temperature, 25 degrees C, to its melting point 49 degrees C
Step-by-step explanation:
Crayon is paraffin wax. The specific heat of paraffin wax is 2.14 J/g°C
As we know

Where
 is the change in temperature
is the change in temperature
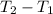
m is the mass of the object
c is the specific heat in J/g°C
Substituting the given values, we get -
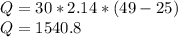 Joules
Joules
1540.8 Joules of energy is required to bring a 30 gram crayon from room temperature, 25 degrees C, to its melting point 49 degrees C