Answer:
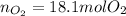
Step-by-step explanation:
Hello there!
In this case, according to the combustion of octane:
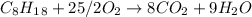
We can see there is a 1:25/2 mole ratio of octane to oxygen; therefore, we can calculate the moles of oxygen via the following stoichiometric factor:
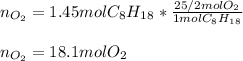
Best regards!