Answer:
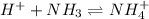
Step-by-step explanation:
Hello there!
In this case, since the buffer is not given, we assume it is based off ammonia, it means the ammonia-ammonium buffer, whereas the ammonia is the weak base and the ammonium ion stands for the conjugate acid. In such a way, when adding HI to the solution, the base of the buffer, NH3, reacts with the former to promote the following chemical reaction:
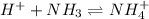
Because the HI is totally ionized in solution so the iodide ion becomes an spectator one.
Best regards!