Answer:
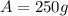
Step-by-step explanation:
Hello there!
In this case, by considering the radioactive decay as a first-order kinetic model:
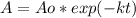
We can firstly calculate the rate constant given the half life:
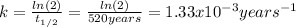
Thus, we can plug in the elapsed time and the initial amount Ao to obtain:
![A=1,000g*exp[-1.33x10^(-3)years^(-1) *(3061-2021)years]\\\\A=250g](https://img.qammunity.org/2022/formulas/chemistry/high-school/a4t55m1sm2jshurhdaicpiauz7cc209k0q.png)
Best regards!