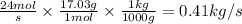Answer:
0.41kg/s
Step-by-step explanation:
Question 5 In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 505. liters per second of dinitrogen are consumed when the reaction is run at 172 °C and the dinitrogen is supplied at 0.88 atm. Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. Clears your work. Undoes your last action. Provides information about entering answers.
Step 1: Convert 172 °C to Kelvin
We will use the following expression.
K = °C + 273.15
K = 172°C + 273.15 = 445 K
Step 2: Calculate the moles of N₂ consumed every second
We will use the ideal gas equation.
P × V = n × R × T
n = P × V/R × T
n = 0.88 atm × 505. L/(0.0821 atm.L/mol.K) × 445 K = 12 mol
Step 3: Calculate the rate of production of ammonia
Let's consider the balanced equation for the synthesis of ammonia.
N₂ + 3 H₂ ⇒ 2 NH₃
The molar ratio of N₂ to NH₃ is 1:2. The rate of production of ammonia is:
12 mol N₂/s × 2 mol NH₃/1 mol N₂ = 24 mol NH₃/s
Step 4: Convert the rate from mol/s to kg/s
We will use the following conversion factors:
- The molar mass of NH₃ is 17.03 g/mol.
- 1 kg = 1000 g