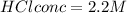Answer:
Step-by-step explanation:
From the question we are told that:
Mass of zinc
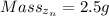
Initial conc of HCl
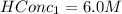
Initial volume of HCl
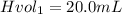
Molar mass of zinc
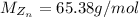
Generally the equation for reaction is mathematically given by

Generally the equation for moles of zinc
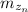 is mathematically given by
is mathematically given by
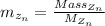
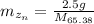
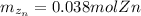
Generally the equation for moles of
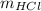 is mathematically given by
is mathematically given by
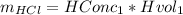
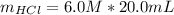
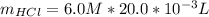
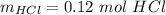
Generally the reacted moles of HCl
![HCl_[reacted]](https://img.qammunity.org/2022/formulas/chemistry/college/huvu0d7k6it53syqn70dn0z060zpbozlxs.png) is mathematically given by
is mathematically given by
Since
Zn:HCl =1:2
Therefore
![HCl_[reacted]=0.038*(2)/(1)](https://img.qammunity.org/2022/formulas/chemistry/college/b72v60doi6uur0foo66als5urbt29wuhwb.png)
![HCl_[reacted]=0.076mol \ HCl](https://img.qammunity.org/2022/formulas/chemistry/college/4o3cavaxe668hb18odw6guizy5lvno8aeq.png)
Generally the moles of HCl after Zn oxidization X is mathematically given by
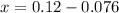
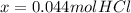
Generally the conc of hydrochloride acid X is mathematically given by
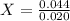
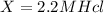
Therefore Conc of HCl