Answer:
2000 L
General Formulas and Concepts:
Atomic Structure
- Moles
- Temperature Conversion: K = °C + 273.15
Gas Laws
Ideal Gas Law: PV = nRT
- P is pressure
- V is volume (in L)
- n is number of moles
- R is gas constant
- T is temperature (in K)
Step-by-step explanation:
Step 1: Define
[Given] 8.8 moles gas
[Given] 0.12 atm
[Given] 56 °C = 329.15 K
Step 2: Solve for V
- Substitute in variables [Ideal Gas Law Formula]:
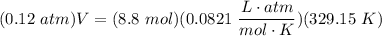
- Isolate V:
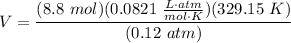
- Multiply/Divide [Cancel out units]:
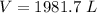
Step 3: Check
Follow sig fig rules and round. We are given 2 sig figs.
1981.7 L ≈ 2000 L