Answer:
33 g.
Step-by-step explanation:
Hello there!
In this case, for these particle-mole-mass relationships problems, it is necessary for us to recall the following equivalence statement, based off the molar mass of the involved compound, C3H8, one mole of particles and the Avogadro's number:
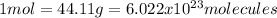
In such a way, we can set up the following expression for the calculation of the mass in the given particles of propane:
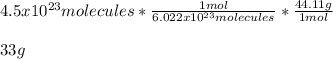
Best regards!