Answer:
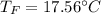
Step-by-step explanation:
Hello there!
In this case, for this calorimetry problem, it is possible for us to realize that the heat lost by the hot silver is gained by the cold far whose specific heat is 3.94 J/g°c, so we can write:
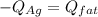
Which can be written in terms of mass, specific heat and temperature as shown below:
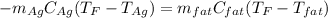
In such a way, solving for the final temperature, we obtain:
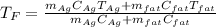
Then, we plug in the given data to obtain:
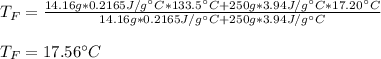
Best regards!