Answer:
B-346.0 mmHg.
Step-by-step explanation:
Hi there!
In this case, according to the Dalton's law, which explains that the total pressure of a gas mixture is calculated by adding up the partial pressure of each gas, we can set up the following equation for the mixture given here:
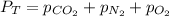
In such a way, for the required answer, it solve for the partial pressure of oxygen to obtain:
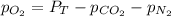
Thereafter, we plug in the known pressures to obtain:
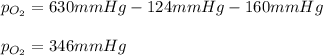
And the answer is B-346.0 mmHg.
Best regards!