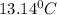Answer: The final temperature of the system will be
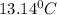
Step-by-step explanation:
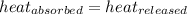
As we know that,
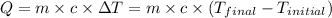
![m_1* c_1* (T_(final)-T_1)=-[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/2iwsj4v7xth00ba9r1k0icno5vnyyj4swj.png) .................(1)
.................(1)
where,
q = heat absorbed or released
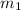 = mass of steam = 25 g
= mass of steam = 25 g
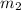 = mass of water = 0.2384 kg = 238.4 g (1kg=1000g)
= mass of water = 0.2384 kg = 238.4 g (1kg=1000g)
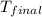 = final temperature = ?
= final temperature = ?
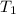 = temperature of steam =
= temperature of steam =
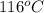
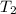 = temperature of water =
= temperature of water =
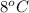
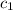 = specific heat of steam =
= specific heat of steam =
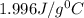
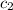 = specific heat of water=
= specific heat of water=
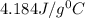
Now put all the given values in equation (1), we get
![25g* 1.996J/g^0C* (T_(final)-116)=-[238.4g* 4.184J/g^0C* (T_(final)-8)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/mw12ol64t7a0xi0sqt00wn6bysg5x60044.png)
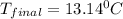
Therefore, the final temperature of the system will be