Answer:
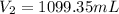
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible to infer that as both the amount of the gas and the pressure remains the same, we can solve this problem via the Charles' law a directly proportional relationship between the volume and temperature:
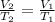
Thus, by solving for the final volume, V2, we obtain:
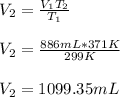
Best regards!