Answer:
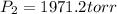
Step-by-step explanation:
Hello there!
In this case, by recalling the Gay-Lussac's gas law, as a directly proportional relationship between pressure and temperature, we can write:
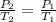
Thus, by solving for the required final pressure, in atmospheres first, we solve for P2 as follows:
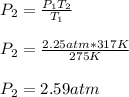
Which in Torricelli is:
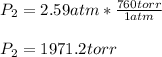
Best regards!