Answer:
[Ar]
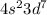
Step-by-step explanation:
To write abbreviated electron configurations, begin with the element of interest and work your way down in atomic numbers to the next smallest noble gas.
Write the noble gas symbol in [brackets] and then write the electron configuration as you would normally.
I hope this helps you
:)