Answer:
The concentration of the 3.2 g of sodium chloride in 8 dm³ of water is 6.845 × 10⁻³ M
Step-by-step explanation:
The concentration of a solution is given as either the ratio of mass to volume, or the ratio of volume to volume or as molarity
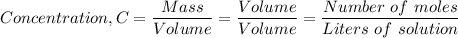
The number of moles present in 3.2 g of sodium chloride, 'n', is given as follows;
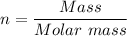
The molar mass of sodium chloride, NaCl = 58.44 g/mol
n = 3.2 g/58.44 g/mol = 0.0547570157 moles
8 dm³ = 8 Liters
Therefore, the concentration, C = 0.0547570157 moles/(8 liters) = 6.845 × 10⁻³ M
The concentration of the 3.2 g of sodium chloride in 8 dm³ of water = 6.845 × 10⁻³ Molar solution.