Answer:
m = 51.93 grams
Step-by-step explanation:
Given that,
Energy added, Q = 747 joules
The specific heat capacity of Aluminium, c = 0.899 J/g°C
The temperature goes from 105.0°C to 121.0°C.
Let the mass of the aluminum sample is m. We know that, the heat added to the system is given by :
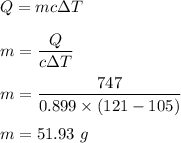
So, the mass of the sample is 51.93 grams.