Answer:

Step-by-step explanation:
A mole is any quantity of a substance that contains 6.02 × 10²³ particles. At standard temperature and pressure, or STP, 1 mole of as is equal to 22.4 liters. This is true for any gas, regardless of the specific kind.
Although it is not specified, we can assume this gas is at STP. Let's set up a ratio using this information: 22.4 L/mol
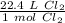
Multiply by the given number of liters: 12
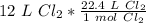
Flip the ratio so the liters of chlorine cancel.
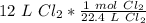
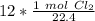
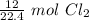
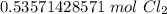
The original measurement of liters has 2 significant figures, so our answer must have the same.
For the number we found, that is the hundredth place.
The 5 in the thousandth place tells us to round the 3 up to a 4.
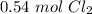
12 liters of chlorine gas at STP is approximately 0.54 moles of chlorine gas.