Answer:
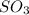
Step-by-step explanation:
Hello there!
In this case, according to the definition of the empirical formula as the smallest representation of the molecular formula of a chemical compound; for us to determine it, we first need to calculate the moles of sulfur in 40.05 g and those of oxygen in 59.95 g as shown below:
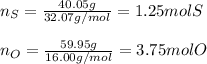
Now, we perform the following mole ratios to figure out the subscripts in the empirical formula, by dividing over the fewest number of moles:
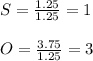
Therefore, the empirical formula turns out:
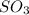
Best regards!