Answer:
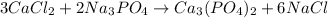
Step-by-step explanation:
Hey there!
In this case, according to the law of conservation of mass, it possible to balance the following reaction:
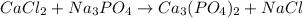
By adding a 3 on CaCl2, a 2 on Na3PO4 and a 6 on NaCl in order to equal the atoms of calcium, chlorine, sodium, phosphorous and oxygen, as follows:
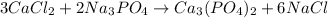
Best regards!