Answer:
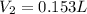
Step-by-step explanation:
Hey there!
In this case, according to the equation for dilution process, in which the moles of the solute remain the same after the addition of additional solvent:
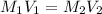
Thus, by solving for the new volume, V2, of the base, NaOH, we can obtain:
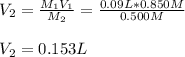
Best regards!