Answer:
n = 4 x 10¹⁸ photons
Step-by-step explanation:
First, we will calculate the energy of one photon in the radiation:
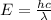
where,
E = Energy of one photon = ?
h = Plank's Constant = 6.625 x 10⁻³⁴ J.s
c = speed of light = 3 x 10⁸ m/s
λ = wavelength of radiation = 567 nm = 5.67 x 10⁻⁷ m
Therefore,
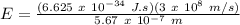
E = 3.505 x 10⁻¹⁹ J
Now, the number of photons to make up the total energy can be calculated as follows:
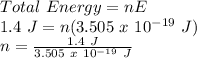
n = 4 x 10¹⁸ photons