Answer:
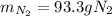
Step-by-step explanation:
Hello there!
In this case, for this stoichiometry-based problem, it is firstly necessary to realize that the decomposition of ammonium dichromate is given by:
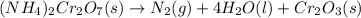
Thus, since the mole ratio between ammonium dichromate and the gaseous nitrogen (molar mass = 28.02 g/mol) is 1:1, we can compute the produced mass of the latter via stoichiometry as shown below:
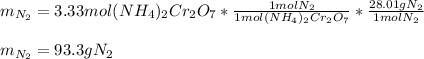
Best regards!