Answer:
237 K
Step-by-step explanation:
Given that,
Initial temperature, T₁ = 201⁰C = 474 K
Let P be the initial pressure. Upon cooling the gas, the pressure decreases by a factor of 2.0, while the volume and the amount of gas remain constant.
Final pressure = P/2
The relation between the pressure and temperature is as follows :
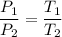
Put all the known values,
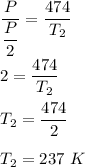
So, the final temperature of the gas is equal to 237 K.