Answer:

Step-by-step explanation:
In a chemical reaction, the reactants are the substances that start the reaction and change into other substances. The results at the end of the reaction are products.
Typically, the reactants are to the left of the arrow, while products are to the right.
We are given this chemical equation:
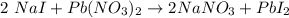
We are asked to find the reactants. Remember, these go into the reaction and are on the left.
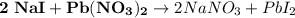
Therefore, the reactants are NaI (sodium iodide) and Pb(NO₃)₂ (lead nitrate). Choice A is correct.