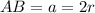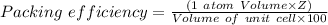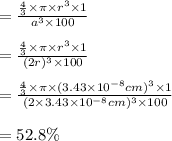Answer:
The answer is "52.8".
Step-by-step explanation:
Please find the graph file in the attachment.
They have the atoms in 8 corners with one unit cell in an one so mesh (SCC).
Atoms throughout the corner contribute
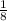 to both the cell unit
to both the cell unit
Atom number per SCC unit cell,
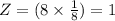
Let 'r' become the atom's radius.
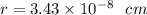
We can see from the diagram that edge length