Answer: 1123000 Joules of energy are required to vaporize 13.1 kg of lead at its normal boiling point
Step-by-step explanation:
Latent heat of vaporization is the amount of heat required to convert 1 mole of liquid to gas at atmospheric pressure.
Amount of heat required to vaporize 1 mole of lead = 177.7 kJ
Molar mass of lead = 207.2 g
Mass of lead given = 1.31 kg = 1310 g (1kg=1000g)
Heat required to vaporize 207.2 of lead = 177.7 kJ
Thus Heat required to vaporize 1310 g of lead =
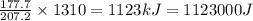
Thus 1123000 Joules of energy are required to vaporize 13.1 kg of lead at its normal boiling point