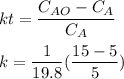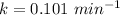Answer:
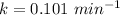
Step-by-step explanation:
From the given information:
The initial concentration of a chemical
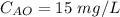
The final concentration is
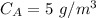 = 5 mg/L
= 5 mg/L
Volume flow rate
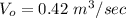
Volume of the tank V = 500 000 L = 500 m³
The time t is determined by using the formula:
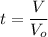
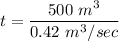
t = 1190.47 sec
t ≅ 19.8 min
∴
The rate of the decay constant is: