Answer:
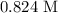
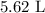
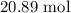
Step-by-step explanation:
Molar mass of NaCl = 58.44 g/mol
Mass of NaCl = 195 g
Volume of solution = 4.05 L
Molarity of solution is
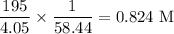
The molarity of the solution is
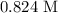
Moles of NaCl = 4.63 mol
Volume is given by
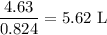
The volume of the solution that contains the required amount NaCl is
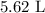
Volume of solution =
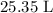
Moles of solution is given by
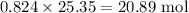
The number of moles of NaCl is
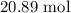 .
.