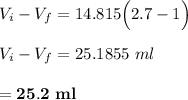Answer:
25.2 ml
Step-by-step explanation:
When the aluminium block is inserted in the container, the overall amount of only the water in the beaker can equal V o.
The weight of the water expelled by the plastic container should be equal to the weight of the aluminium block, according to the buoyancy balance relation.
i.e.
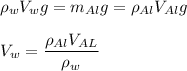
When the aluminium block is inserted into the plastic container, the initial volume of water = 60 ml
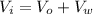
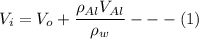
When the aluminium block is placed outside the container, the volume of the water
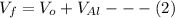
By subtracting equation (1) and (2)
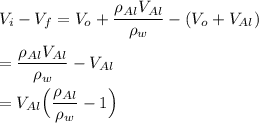
since;
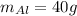
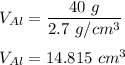
Similarly;
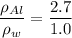
= 2.7