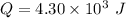Answer:
The solution of the given query is explained below in the explanation portion.
Step-by-step explanation:
Given value is:
Work,
⇒
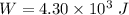
(a)
The change in the internal energy will be:
⇒
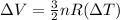
Throughout the isothermal procedure, The temperature will be constant "ΔT = 0".
then,
⇒
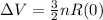
⇒
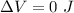
(b)
As we know,
⇒
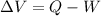
On substituting the value, we get
⇒
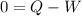
On adding W both sides, we get
⇒

⇒
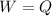
On substituting the given value of "W", we get
⇒