Answer:
When 1.20 mole of ammonia reacts, 1.8 moles of water are produced.
Step-by-step explanation:
The balanced reaction is:
4 NH₃(g) + 5 O₂(g) → 4 NO (g) + 6 H₂O
By stoichiometry of the reaction, the following amounts of moles participate in the reaction:
- NH₃: 4 moles
- O₂: 5 moles
- NO: 4 moles
- H₂O: 6 moles
Then you can apply the following rule of three: if by stoichiometry 4 moles of ammonia produce 6 moles of water, 1.2 moles of ammonia will produce how many moles of water?
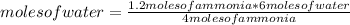
moles of water= 1.8 moles
When 1.20 mole of ammonia reacts, 1.8 moles of water are produced.