Answer:
I. When the temperature is 330K, the volume is 0.025 m³
II. When the volume is 0.0022 m³, the temperature is 300 K.
Step-by-step explanation:
Charles states that when the pressure of an ideal gas is kept constant, the volume of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Charles is given by;
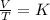
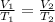
Where;
V1 and V2 represents the initial and final volumes respectively.
T1 and T2 represents the initial and final temperatures respectively.
From the graph, we can deduce the following;
I. When the temperature is 330K, the volume is 0.025 m³
II. When the volume is 0.0022 m³, the temperature is 300 K.